Monday, November 30, 2009 7:15 PM
11/30/09 - DAY 1ACONCENTRATIONSolution - A homogenous mixture
Solute - The one present in a smaller amount
Solvent - The one present in a greater amount
Concentration - (Amount of Solute)/(Amount of Solvent)
SOME UNITS FOR CONCENTRATION
- g/mL
- g/L
- mg/L
- mg/mL
- microgram/L
The most common and useful units are
mol/L - Molarity - Molar Concentration
- M = mol/L
- mol = (M)(L)
- L = mol/M
EXAMPLES:
- Stephanie dissolves 40.0 g of NaOH is enough water to make 200 mL of the solution. What is the concentration?
- Concentration = 40.0 g/200mL = 0.200 g/mL
- V=0.200L
- NaOH = M = 1.0 mol/0.200 L = 5.0mol/L OR 5.0 M
- Jordan wants to make 600.0 mL of 0.60 M CaCl2. What mass of solid CaCl2 is required?
- mol = 0.60 M(0.600L)
- mol = 0.36 mol
- 0.36 mol x (111.1g/mol) = 39.996 g
- Mass = 40.0 g
WATCH THIS VIDEO
Wednesday, November 18, 2009 5:27 PM

Today was the last class before our chemistry mid-term exam! yikes!
We went over homework from last class ( homework question sheet # 22-24). As well, we got an outline and review pack for the exam. good luck everyone!
4:36 PM
EMPIRICAL FORMULAS!
The difference between a molecular formula and an empirical formula is that the molecuar gives you the actual number; for example: Pb2O4. An empirical formula however, gives you the number ratios of the elements in the compound; Pb2O4 would become PbO2.
Example:-What is the empirical formula for 15.9% of B and 84.1% of F?
Step 1: create a chart like the following:
ELEMENT: B
MASS (g): 15.9
ATOMIC MASS: 10.8
MOLES: 15.9/10.8= 1.5 moles
MOLES/SMALLEST MOLE: 1.5/1.5= 1
RATIO: 1
ELEMENT: F
MASS (g): 84.1
ATOMIC MASS: 19.0
MOLES: 84.1/19.0= 4.4 moles
MOLES/SMALLEST MOLE: 4.4/1.5= 2.93333
RATIO: 3
The empirical formula is BF3.
Step 2: once you have set up the chart you start to fill it in. the elements are Boron and Fluorine, their masses are 15.9 g and 84.1 g, their atomic mass is 10.8 and 19.0, and they have 1.5 moles and 19.0 moles. when you get to the moles/ smallest mole section you simply look at the number of moles each element has B= 1.5 mol, and F= 4.4 mol. boron has the smallest mol, therefore you divide both moles by 1.5. if you do not get a whole number then you either round it or multiply it by 1, 2, 3, 4 etc... until you get one. but remember if you multiply one of them then you have to multiply the rest by the same number.
Go to this site to see a video:
http://www.youtube.com/watch?v=r2Log6-voWo
Monday, November 16, 2009 7:26 PM
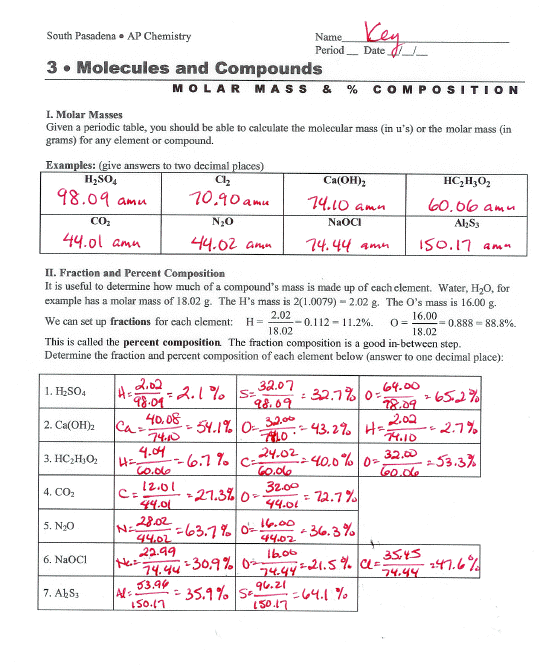
11/12/09 - DAY 1CDABPercent Mass of Elements in Compounds- Find the % carbon by mass in Ethane (C2H6)
- 2(12g/mol) + 6(1.0g/mol) = 30 g/mol
- % Carbon = 24g/30g = 80.0 %
- % Hydrogen = 6g/30g = 20.0 %
Percent Composition- Means the % mass of each element in a compound
- Find the % Composition of K2Cr2O7
- 2(39.1g/mol) + 2(52.0g/mol) + 7(16.0g/mol) = 294.2 g/mol
- % K = 78.2/294.2 x 100 = 26.6 %
- % Cr = 104/294.2 x 100 = 35.4 %
- % O = 112/294.2 x 100 = 38.1 %
Finding the mass of an element in a given of a compound
- Find the mass of Carbon combined in a 25.0 g sample of CO2
- % O = 72.2 %
- % C = 27.3 % > (0.273)(25.0g) = 6.82 g
- Find the mass of K,C, and O contained in K2CO3. If the sample is 450.0 g
- 2(39.1g/mol) + 3(16.0g/mol) + 12 = 138.2 g/mol
- % K = 56.6 % > (0.566)(450g) = 250.2 g
- % C = 8.7 % > (0.087)(450g) = 39.15 g
- % O = 34.7 % > (0.347)(450g) = 156.15 g

6:39 PM
11/10/09 - DAY 1A
Density--(D= M/V)--Mass--(molar mass)--Moles--(6.02 x 10^23)--MoleculesMass--( x 22.4 L)--Volume(@STP)Molecules--( x subscripts)--Atoms1. 1.25L of an unknown gas has a mass of 3.47g. What is the molar mass if it is at STP?1.25L x (1mol/22.4L) =0.0558mol 3.47g/0.0558mol=62.2g/mol
2. 250mL of a gas which is known to contain one sulphur atom and an unknown number of fluorides has a mass of 1.63 at STP. Find the molar mass (g/mol)250mL x (1L/1000mL) = 0.25L x 1mol/22.4L = 0.0111mol
1.63g/0.0111mol = 146.85g/mol
Here are a couple examples from us:
1. Find the mass of 2.5mol of NO2
1N: 1(14)=14>>2O: 2(16)=32
= 46g/mol
NO2 ---> 46g/mol--->mol/46g(1/2.5) = 1/115g (reciprocal)
115g
2. Find the volume occupied by 0.060mol of I2 at STP0.060mol x 22.4L/1mol = 1.34L

Sunday, November 1, 2009 3:39 PM
Atoms and MoleculesFor monoatomic elements
a molecule = an element
(Ne) = (Ne)
Diatomic elements:
Molecule = Element
Cl2 = Cl
Molecules of compounds
H--H
|O|
Molecule
2 'H' atoms in 1 molecule
1 'O' atom in 1 molecule
Example: Write Ammonium Carbonate
Answer: (NH4)2CO3
N: 2
H: 8
O: 3
C: 1
Moles<>Molecules6.02 x 10^23 molecules/ 1 mol
or 1 mol/ 6.02 x 10^23 molecules
Example: How many molecules are in 0.25 mol of CO2
Answer: 0.25 mol x 6.02 x 10^23 molec/1 mol = 1.505 x 10^23 molec.
Example: 5.1772 x 10^24 molecules of H2O = ? moles
Answer: 5.1772 x 10^24 molec x 1 mol/ 6.02 x 10^23 molec = 8.6 mol
Example: Find the number of 'H' atoms in 4.0 mol of ammonia? [moles->molecules-> H atoms]
4.0 mol =x3 molecules
Answer: 4.0 mol x 6.02 x 10^23/ 1 mol = 2.41 x 10 ^24 molecules x3 = 7.22 x 10^24 'H' atoms




